A&E and COVID ward (days 0-3)
A 65-year-old male (Tony) was brought to A&E by his wife due to increasing dyspnea and a persistent dry cough. He had been feeling “under the weather” for the past week and felt “quite breathless at times”. He had also noticed some abdominal pain and mild diarrhoea in the last few days.
On clinical examination, it was found that Tony was hypoxemic (O2 sat = 89%) and had a fever of 39°C. A nasopharyngeal swab sample was taken for PCR diagnosis of SARS-CoV-2 which came back positive. His notes show that he has T2DM that is well controlled with long-acting insulin and lifestyle adjustments.
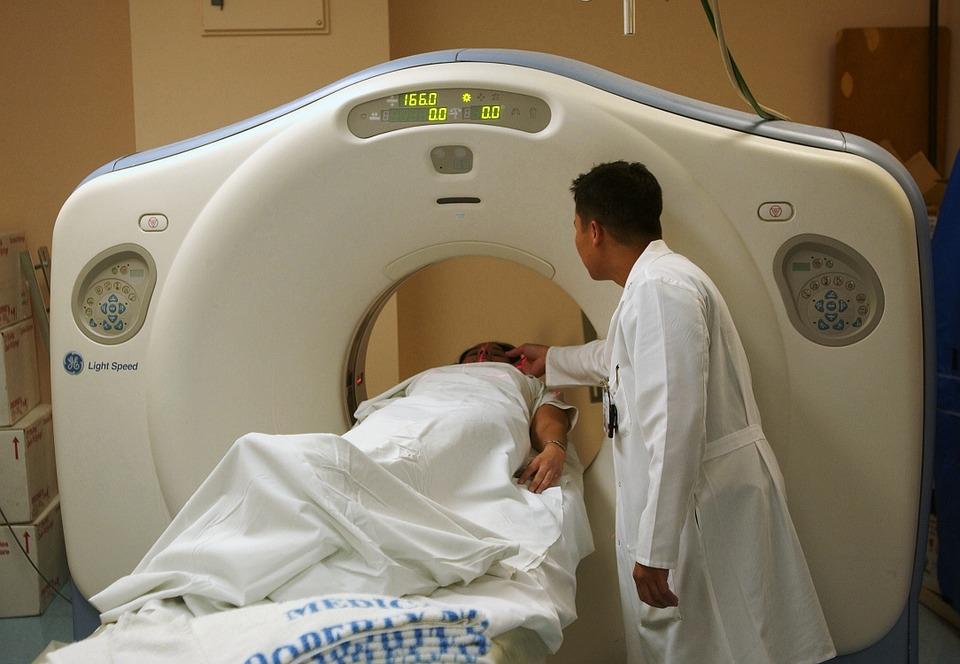
Tony was admitted to a COVID ward for monitoring and received oxygen via high-flow nasal cannula. Unfortunately, his condition deteriorated over the next 3 days, and he remained febrile despite receiving antibiotics. A chest CT scan showed multiple bilateral ground-glass opacities with a crazy-paving appearance and many peripheral nodular consolidations. On day 3 his breathing became laboured and he had a PaO2:FiO2 ratio of 136 mm Hg (moderate ARDS), so was moved to ICU.
Patient notes
| Referred from | A&E/wards |
| Diagnoses |
|
| Demographics |
|
| Recent radiology | Chest CT showed multiple bilateral ground-glass opacities with a crazy-paving appearance and many peripheral nodular consolidations. |
In the ICU (days 3-7)
Tracheal aspirates (TA) were obtained at the time of admission to the ICU, which were tested for galactomannan and fungal cultures (both negative). Blood cultures and serum BDG were also negative at this time.
He was intubated and treated with corticosteroids and more systemic antibiotics, which seemed to gradually improve his condition. However, on day 7 his fever returned and his breathing deteriorated significantly, with traces of blood appearing in his sputum. Chest CT findings were inconclusive as to whether a bacterial/fungal superinfection is present. As he has already received antibiotics, a fungal infection is suspected but no obvious source of bloodstream infection is apparent (e.g. CVS).
Which tests would you order?
Blood cultures
Correct
Cultures are important for detecting invasive infections such as candidaemia, although moulds like Aspergillus are rarely isolated from blood.
Serum BDG
Correct
Beta-D-glucan is a fungal cell wall antigen produced by Aspergillus, Candida and Pneumocystis.
Two consecutive positive results for serum BDG provides mycological evidence for probable invasive aspergillosis in the ICU
N.B. other causes of elevated serum concentration need to be excluded as BDG is not specific for Aspergillus
Galactomannan
Correct
Galactomannan is a fungal cell wall antigen produced by Aspergillus (but NOT Candida).
A galactomannan antigen index of >0.5 in plasma or serum, and/or galactomannan antigen >0.8 in BAL fluid provides mycological evidence for probable invasive aspergillosis in the ICU.
Respiratory cultures
Correct
While fungal culture is not always a particularly sensitive test, it is very important for identification and susceptibility testing.
CrAg test
Incorrect
Cryptococcal antigen testing (CrAg) is unlikely to help as Cryptococcus is rare in the UK. This patient is not known to be HIV+ or neutropaenic, or showing neurological symptoms suggesting cryptococcal meningitis.
Aspergillus LFD (rapid antigen test)
Correct (if available)
A commercial lateral flow test (point-of-care test) has been developed that gives a rapid (30 min) result on the ward. If your hospital uses it, this can help in making a quick decision whether to commence antifungals.
Thresholds are comparable to ELISA galactomannan testing of bronchoalveolar
lavage and serum.
Aspergillus PCR
Correct
Two positive aspergillus PCR results provide mycological evidence of probable invasive aspergillosis in the ICU setting.
Direct (KOH) microscopy
Incorrect
It is unlikely that enough fungal material would be present to visualise
Aspergillus fumigatus precipitins
Incorrect
This test measures antibodies against Aspergillus, which is relevant to chronic conditions
In the ICU (days 7-9)
On day 7, his TA galactomannan and Aspergillus PCR were positive, although all blood and respiratory cultures were still negative. While this is not sufficient for a firm diagnosis of invasive fungal infection, a pragmatic decision was made to treat this patient as if he had COVID-associated pulmonary aspergillosis (CAPA) as he was deteriorating rapidly.
Tony was started on isavuconazole with advice from the infectious diseases pharmacist. Note that voriconazole was not used as it is frequently associated with major drug-drug interactions in the ICU setting. As part of the hospital antifungal stewardship programme, his response was monitored closely and the tests were repeated on days 9 and 12.
Summary of test results
| Test | Day 3* | Day 7 | Day 9 |
| Respiratory cultures | Negative | Negative | Negative |
| Serum BDG | Negative | Negative | Negative |
| TA cultures | Negative | Negative | Negative |
| TA galactomannan | Negative | Positive | Negative |
| Aspergillus PCR | Not done | Positive | Negative |
*Admitted to ICU
What could his diagnosis be?
COVID-associated pulmonary aspergillosis (CAPA)
This is possible
Presence of galactomannan and Aspergillus DNA in tracheal aspirates is consistent with CAPA, but not sufficient to make a firm diagnosis
Mucormycosis
Less likely
Moulds causing mucormycosis do not produce galactomannan
Candidaemia
Less likely
Candidaemia would be expected to produce a positive blood culture and beta-D-glucan test
In the ICU (days 9-12)
Tony did not show significant improvement in response to the isavuconazole and remained intubated. On day 9, the TA galactomannan and Aspergillus PCR were both negative, suggesting that the day 7 results were most likely false positives, possibly due to contamination of samples with Aspergillus from the oral microbiota. Serum BDG was also negative at all time points. It was recommended by the ID pharmacist to stop the isavuconazole treatment.
BAL fluid collected by bronchoscopy on day 12 grew Actinomyces, an unusual slow-growing bacterium that can rarely cause pneumonia in immunocompetent patients (Eschapasse et al, 2017). Antibiotics were administered and Tony recovered enough to be stepped down to a normal ward after another 2 weeks. He will be referred for pulmonary rehab when he is discharged.
Long-term recovery

Luckily Tony survived, but his discharge from hospital is only the start of a long road to his recovery.
Long COVID
Some people experience symptoms for many months after recovering from COVID. Most commonly fatigue but can also include palpitations, tinnitus, nausea, ‘brain fog’ and joint pain. Generate a referral to the Your COVID Recovery programme
Post-Intensive Care Syndrome
As well as trouble with breathlessness and fatigue, many patients will have problems with swallowing, speaking, sleep and concentration while recovering from a critical illness
- NHS guide for patients / NHS guide for carers
- ICUsteps.org produces a wide range of downloadable resources for patients and their families, including guides for children and versions in 19 different languages
Mental health
Most people will experience some degree of low mood and worry after a critical illness, which in some cases can develop into clinical depression, anxiety or PTSD.
- Make sure he knows how to self-refer to his local IAPT service or find a private therapist
- Direct him to Books on Prescription and NHS self-help guides
- Direct him to the local and online support groups run bu ICUsteps.org
All cases of suspected CAPA should be managed in accordance with local hospital guidelines, in close consultation with the infectious diseases team. Prompt antifungal treatment saves lives, but prescribing antifungals when they are not needed is a major cause of antifungal resistance and risks serious toxicity / drug interactions.
ECMM / ISHAM 2021 consensus criteria
During the first wave of the pandemic, incidence rates of invasive aspergillosis in COVID patients between 8% and 33% were reported, largely as a result of misinterpretation of fungal diagnostics. This was particularly true during the first wave of COVID as many centres would not allow bronchoscopy due to the risk of aerosolizing virus particles. Figures published by the UK National Mycology Reference Laboratory in Bristol suggested the true incidences in the UK were closer to 5% proven CAPA and 15% possible CAPA (Borman et al, 2021).
Later a consensus statement (Koehler et al, 2021) was published in Lancet Infectious Diseases with a detailed explanation of the criteria used to define possible, probable and proven CAPA. They recommended voriconazole or isavuconazole as first-line therapy, or liposomal amphotericin B if azole resistance is a concern. Therapeutic drug monitoring is recommended for azole antifungals and may need to be ordered from a mycology reference laboratory such as MRCM
In practice, patients may be ‘treated as CAPA’ rather than given a formal diagnosis of proven, probable or possible CAPA. It is also important to note that rates may be different between healthcare systems or settings, so it is important to contact your local infectious diseases team. A diagnostic algorithm (White et al, 2020) for use in Wales was devised and tested by a group led by the Mycology Reference Centre in Cardiff.
Why is it so hard to diagnose invasive fungal infections?
Invasive fungal infections are life-threatening and sometimes a treatment decision must be made before all test results are available. Therefore guidelines generally classify cases by degree of certainty (e.g. proven/probable/possible). Radiological appearance and test results can differ depending on stage of infection (airway invasive vs angioinvasive) or patient characteristics (e.g. neutropaenia).
The EORTC/MSGERC definitions of invasive pulmonary aspergillosis (IPA) were originally designed for use in clinical trials, rather than clinical diagnosis. They were based around immunosuppressed patients with cancer or who had received an HSCT/organ transplant. These guidelines were recently updated (Donnelly et al, 2020) to include Aspergillus PCR and also to make them suitable for immunocompetent patients.
An algorithm called AspICU was designed for use critical care settings (Blot et al, 2011).
Invasive mucormycosis
Another fungal coinfection that received much attention during the pandemic is invasive mucormycosis, or ‘black mould’, which is caused by various mould species (Mucor, Rhizopus, etc). This was a widespread problem in India, but is very rare in the UK. Unlike Aspergillus, these species do not produce BDG in their cell walls. They are even more aggressively invasive than Aspergillus and the infection is usually treated with a combination of surgery and antifungals, albeit with a very poor survival rate.
- Indian consensus guidelines: Muthu et al, 2022

